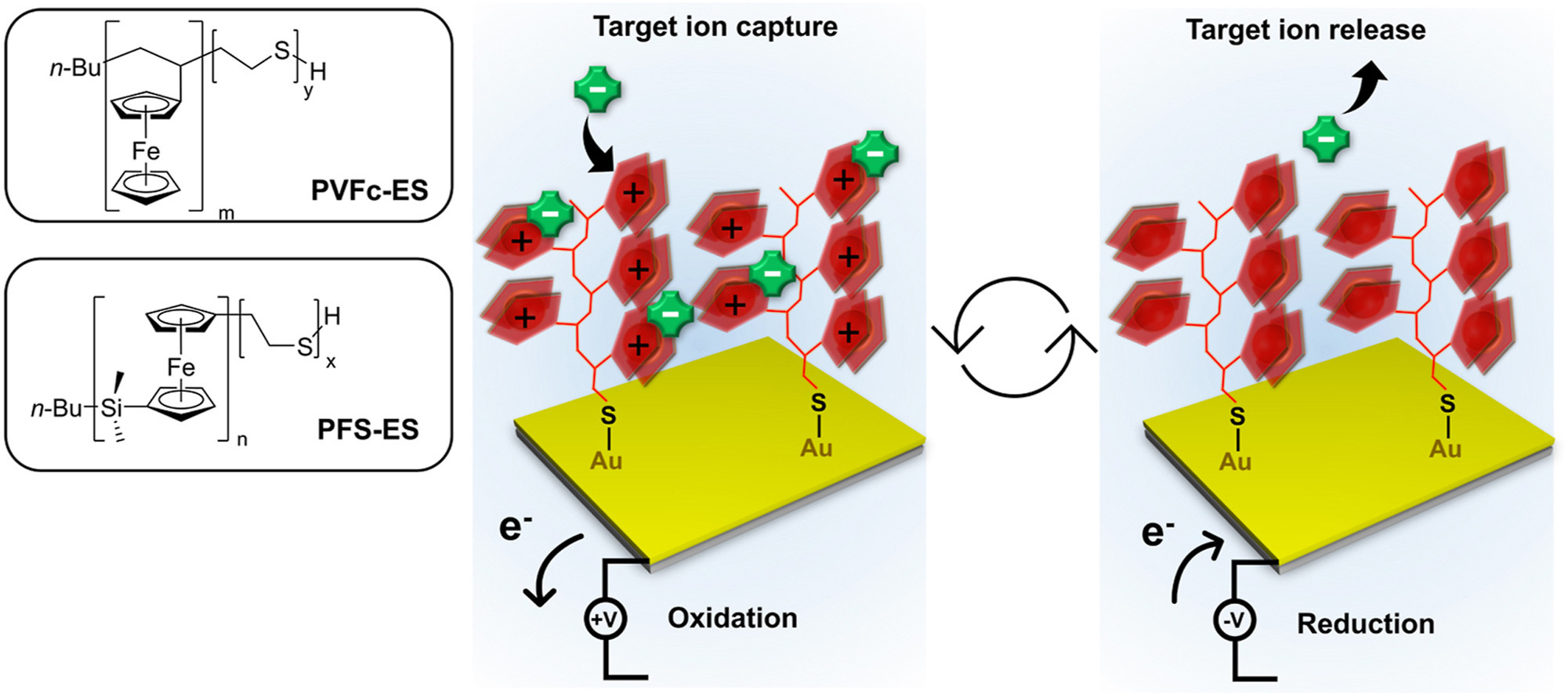
Interfacial charge storage mechanisms of composite electrodes based on poly(ortho-phenylenediamine)/carbon nanotubes via advanced electrogravimetry
Authors: El Mahdi Halim, Rezan Demir-Cakan, Hubert Perrot, Mama El Rhazi, Ozlem Sel
Journal: The Journal of Chemical Physics (2022)
Abstract
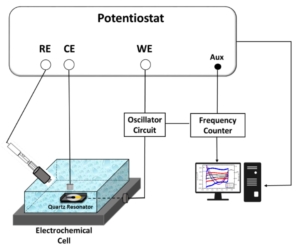
To reach a deeper understanding of the charge storage mechanisms of electrode materials is one of the challenges toward improving their energy storage performance. Herein, we investigate the interfacial ion exchange of a composite electrode made of carbon nanotube/poly( ortho-phenylenediamine) (CNT/P oPD) in a 1M NaCl aqueous electrolyte via advanced electrogravimetric analyses based on electrochemical quartz crystal microbalance (EQCM). Classical EQCM at different scan rates of the potential revealed the complex electrogravimetric behavior likely due to multi-species participation at different temporal scales. Thereafter, in order to better understand the behavior of each species (ions, counter ions, and co-ions) in the charge compensation mechanism, the electrogravimetric impedance spectroscopy analysis (also called ac-electrogravimetry) was pursued. Ac-electrogravimetry revealed the role of each species where Na + cations and Cl − anions as well as protons participate in the charge compensation mechanism of the CNT/P oPD composite with different kinetics and proportions. The water molecules with opposite flux direction with the cations are also detected, suggesting their exclusion during cationic species transfer. Having analyzed ac-electrogravimetry responses in depth, the synergistic interaction between the CNT and P oPD is highlighted, revealing the improved accessibility of species to new sites in the composite.
You may read the full paper here.
Interfacial charge storage mechanisms of composite electrodes based on poly(ortho-phenylenediamine)/carbon nanotubes via advanced electrogravimetry