Potentiostat Integration Application Note
March 15th 2021: AWSensors is pleased to invite you to take a look to the its new Application Note on Potentiostat Integration entitled “Potentiostat integration with AWSensors equipment“.
Summary of the Note
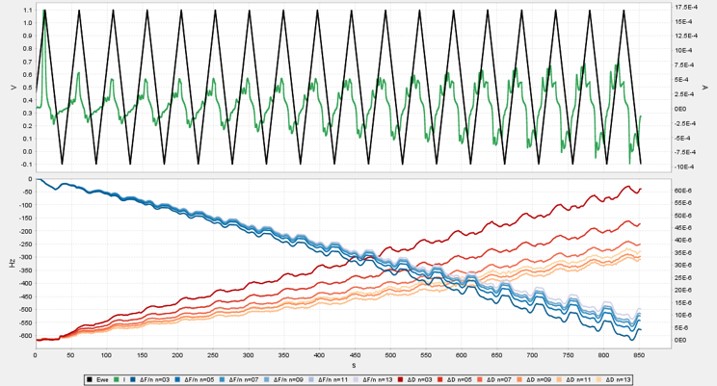
Seamless integration of QCMD and electrochemistry in the AWSensors EQCMD systems allows simultaneous measurements of the amount, electrochemical properties, and organization of material at the air/liquid interface accessed through the changes in the resonance frequency and dissipation measured by QCMD and the potential/current relationships measured with an integrated potentiostat or a galvanostat. The capabilities of the integrated AWSensors electrochemical EQCMD systems are illustrated here using the electropolymerization of aniline as an example.
Introduction
Investigation of complex interfacial processes benefit from combinations of complementary surface-analytical techniques that are based on different principles and approach the interface from complementary perspectives. [1,2] In this regard, the electrochemical quartz crystal microbalance with dissipation measurements, or EQCMD, has a venerable history. [3-5] Indeed, one of the incentives for immersing QCMD in liquids in the 1980s was to perform combined EQCMD measurements [6]. The two approaches are complementary in terms of the information they provide about the solid-liquid interface: interfacial mass transfer and structural changes are accessed with QCMD, while electrochemistry is concerned with the interfacial charge transfer and surface potential changes. Of particular interest is the quantitative characterization of electrochemically driven structural or viscoelastic transitions in interfacial layers, e.g., in battery research [7].
To accommodate the needs of researchers working with electrochemical applications in a wide variety of fields, AWSensors developed QCMD instruments with integrated potentiostat/galvanostat control for synchronizing QCMD and electrochemical experiments with appropriate BioLogic potentiostat or galvanostat. To illustrate the functionality, we use a straight-forward aniline polymerization experiment.
Electropolymerization of aniline to form polyaniline (PANI), and its electrochemical properties, have been widely studied, including by electrochemical quartz crystal microbalance (ref. [8-10], and references therein). Here, we go through the necessary steps for setting up an electrochemical experiment with the integrated QCMD/potentiostat combination for following aniline electropolymerization on the gold electrode surface of a QCMD sensor. The gravimetric and electrochemical results are presented, and the gravimetric results are compared with the cyclic voltammetry.
Continue reading by downloading the full Note (below) …
Download Full Note
You can download the full Application Note in pdf file from this link or download it from our Applications Web Page where you can find this and the rest of our Technology Notes.
Other references about the Note