Journal: ACS Appl. Nano Mater. (2021), 4, 5, 4964–4973
Authors: Adnane Bouzina, Hubert Perrot, Ozlem Sel, Catherine Debiemme-Chouvy.
Abstract:
Graphene-based composites are promisin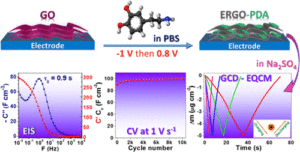 g materials for supercapacitors due to the high specific surface area and electrical conductivity of graphene. Reduction of graphene oxide (GO) is a practical approach to obtain a graphene-like material, but it suffers from restacking of the graphene sheets. Herein, a two-dimensional composite electrode based on electrochemically reduced GO (ERGO) and polydopamine (PDA) is reported, where PDA is used as a “bioinspired chemical insert” to tackle with the restacking issue of graphene layers. This green and facile electrochemical fabrication method starts from electroreduction of GO followed by electro-oxidation of dopamine (DA), present in the same electrolyte, by a simple switch between a cathodic and an anodic potential. The optimized ERGO-PDA composite electrode possesses combined features of excellent capacitive behavior, with a relaxation time (τ0) of 0.88 s, high gravimetric and volumetric capacitances (178 F·g–1 and 297 F·cm–3, respectively, at 10 mV·s–1), and finally an excellent cycling stability at 100–2000 mV·s–1 at least for 30,000 cycles. The DA electropolymerization yield monitored by a quartz crystal microbalance and X-ray diffraction measurements demonstrate that PDA is formed between the graphene sheets which prevents the sheets from restacking and facilitates species diffusion inside the composite, leading to a volumetric energy density of 8.6 mWh·cm–3 for a power density of 7.8 W·cm–3. Additionally, the electrochemical quartz crystal microbalance demonstrates a dominant cationic charge compensation and a very efficient interfacial transfer characteristic since a totally reversible mass response during charge/discharge was observed for the optimized ERGO-PDA electrode.
g materials for supercapacitors due to the high specific surface area and electrical conductivity of graphene. Reduction of graphene oxide (GO) is a practical approach to obtain a graphene-like material, but it suffers from restacking of the graphene sheets. Herein, a two-dimensional composite electrode based on electrochemically reduced GO (ERGO) and polydopamine (PDA) is reported, where PDA is used as a “bioinspired chemical insert” to tackle with the restacking issue of graphene layers. This green and facile electrochemical fabrication method starts from electroreduction of GO followed by electro-oxidation of dopamine (DA), present in the same electrolyte, by a simple switch between a cathodic and an anodic potential. The optimized ERGO-PDA composite electrode possesses combined features of excellent capacitive behavior, with a relaxation time (τ0) of 0.88 s, high gravimetric and volumetric capacitances (178 F·g–1 and 297 F·cm–3, respectively, at 10 mV·s–1), and finally an excellent cycling stability at 100–2000 mV·s–1 at least for 30,000 cycles. The DA electropolymerization yield monitored by a quartz crystal microbalance and X-ray diffraction measurements demonstrate that PDA is formed between the graphene sheets which prevents the sheets from restacking and facilitates species diffusion inside the composite, leading to a volumetric energy density of 8.6 mWh·cm–3 for a power density of 7.8 W·cm–3. Additionally, the electrochemical quartz crystal microbalance demonstrates a dominant cationic charge compensation and a very efficient interfacial transfer characteristic since a totally reversible mass response during charge/discharge was observed for the optimized ERGO-PDA electrode.