Journal: ACS Energy Lett. (2021), 6, 7, 2638–2644.
Authors: Amey Nimkar, Fyodor Malchick, Bar Gavriel, Meital Turgeman, Gil Bergman, Tianju Fan, Shaul Bublil, Reut Cohen, Michal Weitman, Netanel Shpigel, Mikhael D Levi, and Doron Aurbach.
Abstract:
Among the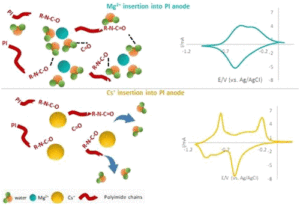 examined organic electrodes for aqueous mono and multivalent ions batteries, polyimide is considered a promising candidate because of its high capacity and good cyclability in different electrolyte solutions. While most of the studies so far were focused on the energetic performance of polyimide anodes, much less is known about their charge storage mechanism and particularly how such electrodes are affected by the solvation properties of the inserted cations. Using in situ EQCM-D, a direct assessment of the cationic fluxes and their hydration shells inserted/extracted to/from PI electrodes upon potential application was performed for a large variety of mono and multivalent cations. Our observations demonstrated a pronounced withdrawal of water molecules from the polymeric electrodes during insertion of chaotropic cations and significantly less water withdrawal upon insertion of kosmotropic cations. These findings are well correlated with the capacity and the rate capability of the polyimide electrodes in the examined systems and shed light on their charge storage mechanism.
examined organic electrodes for aqueous mono and multivalent ions batteries, polyimide is considered a promising candidate because of its high capacity and good cyclability in different electrolyte solutions. While most of the studies so far were focused on the energetic performance of polyimide anodes, much less is known about their charge storage mechanism and particularly how such electrodes are affected by the solvation properties of the inserted cations. Using in situ EQCM-D, a direct assessment of the cationic fluxes and their hydration shells inserted/extracted to/from PI electrodes upon potential application was performed for a large variety of mono and multivalent cations. Our observations demonstrated a pronounced withdrawal of water molecules from the polymeric electrodes during insertion of chaotropic cations and significantly less water withdrawal upon insertion of kosmotropic cations. These findings are well correlated with the capacity and the rate capability of the polyimide electrodes in the examined systems and shed light on their charge storage mechanism.




