Ion Dynamics at the Carbon Electrode/Electrolyte Interface: Influence of Carbon Nanotubes Types
Authors: Freddy Escobar-Teran, Hubert Perrot and Ozlem Sel.
Journal: Materials (2022)
Abstract
Electrochemical quartz crystal microbalance (EQCM) and AC-electrogravimetry methods were employed to study ion dynamics in carbon nanotube base electrodes in NaCl aqueous electrolyte. Two types of carbon nanotubes, Double Wall Carbon Nanotube (DWCNT) and Multi Wall Carbon Nanotube (MWCNT), were chosen due to their variable morphology of pores and structure properties. The effect of pore morphology/structure on the capacitive charge storage mechanisms demonstrated that DWCNT base electrodes are the best candidates for energy storage applications in terms of current variation and specific surface area. Furthermore, the mass change obtained via EQCM showed that DWCNT films is 1.5 times greater than MWCNT films in the same potential range. In this way, the permselectivity of DWCNT films showed cation exchange preference at cathode potentials while MWCNT films showed anion exchange preference at anode potentials. The relative concentration obtained from AC-electrogravimetry confirm that DWCNT base electrodes are the best candidates for charge storage capacity electrodes, since they can accommodate higher concentration of charged species than MWCNT base electrodes.
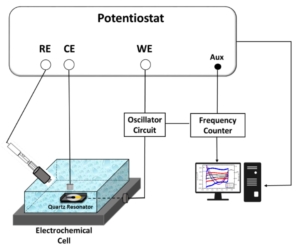
You may read the full paper here.
Ion Dynamics at the Carbon Electrode/Electrolyte Interface: Influence of Carbon Nanotubes Types



