Fouling of Reverse Osmosis Membrane with Effluent Organic Matter: Componential Role of Hydrophobicity
Authors: Noa Stein, Revital Sharon-Gojman, Meagan S. Mauter, Roy Bernstein and Moshe Herzberg
Journal: ACS ES&T Water (2023)
Abstract
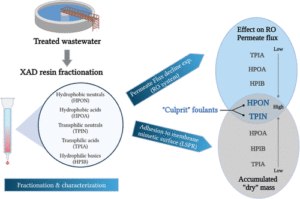
Organic matter dissolved in tertiary effluents (effluent organic matter, EfOM) is the predominant organic membrane foulant in tertiary wastewater reverse osmosis (RO) desalination, ultimately causing biofouling. The interrelated effects of EfOM fractions of different hydrophobicity and polarity on membrane performance were studied by (i) examining each fraction’s overall effect on membrane permeability; (ii) analyzing the intrinsic hydraulic resistance induced by each fraction; (iii) studying their adsorption on the active layer of an RO membrane using a quartz crystal microbalance with dissipation monitoring (QCM-D); (iv) assessing their “dry” molecular mass when adsorbed on polyamide using localized surface plasmon resonance (LSPR) sensing; (v) analyzing their hydrodynamic radii by dynamic light scattering (DLS); and (vi) characterization using excitation–emission matrix (EEM) analysis and parallel-factor (PARAFAC) modeling. Hydrophobic and transphilic neutral fractions (containing ∼12.5% total organic carbon) have the greatest effect on membrane flux reduction and the highest hydraulic resistance and adhere most strongly to polyamide surfaces, resulting in the highest adsorbed “dry” mass. Therefore, in terms of their effect on RO permeate flux reduction, these fractions are the most detrimental in the EfOM mix. EEM analysis and associated PARAFAC modeling indicate that the main components causing this effect are mixtures of protein-like compounds, together with humic-like substances. Novel LSPR-based analysis elucidated the role of the fractions most detrimental to membrane permeability through measurement of dry mass surface concentration on a polyamide mimetic sensor. This study provides valuable insights into the roles of different EfOM fractions in RO membrane fouling and enhances our understanding of fouling during tertiary wastewater desalination.
You may read the full paper here.
Fouling of Reverse Osmosis Membrane with Effluent Organic Matter: Componential Role of Hydrophobicity





