Quartz crystal microbalance with dissipation monitoring for studying soft matter at interfaces
Authors: Diethelm Johannsmann & Ilya Reviakine
Journal: Nature Reviews Methods Primers
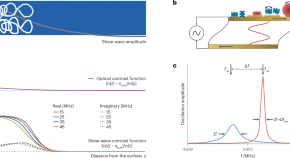 Abstract: Quartz crystal microbalance with dissipation monitoring (QCM-D) probes interfaces by subjecting them to a periodic shear stress exerted by an acoustic resonator. The changes in the resonance frequency, Δf, and the half-width at half-maximum of the resonance, ΔΓ (closely related to the changes in the dissipation, ΔD), measured with the QCM-D are proportional to the in-phase and out-of-phase components of the area-averaged transverse stress at the resonator surface, respectively. Amounts, organization and properties of soft matter at an interface between the resonator and a liquid or a gas are derived from the measurements of Δf and ΔΓ on multiple overtones at megahertz frequencies. The properties include viscoelasticity and stress relaxation dynamics on the timescale of the oscillation period. This Primer offers guidelines on instrument design, experimental procedures and data analysis for interpreting frequency and bandwidth changes in terms of structure and dynamics of the sample. There is a focus on recent progress in the analysis of the acoustic ratio, ΔΓ/(−Δf), and numerical methods of modelling. Limitations of the existing approaches for data analysis are discussed. Challenges and possible future developments are formulated in an outlook.
Abstract: Quartz crystal microbalance with dissipation monitoring (QCM-D) probes interfaces by subjecting them to a periodic shear stress exerted by an acoustic resonator. The changes in the resonance frequency, Δf, and the half-width at half-maximum of the resonance, ΔΓ (closely related to the changes in the dissipation, ΔD), measured with the QCM-D are proportional to the in-phase and out-of-phase components of the area-averaged transverse stress at the resonator surface, respectively. Amounts, organization and properties of soft matter at an interface between the resonator and a liquid or a gas are derived from the measurements of Δf and ΔΓ on multiple overtones at megahertz frequencies. The properties include viscoelasticity and stress relaxation dynamics on the timescale of the oscillation period. This Primer offers guidelines on instrument design, experimental procedures and data analysis for interpreting frequency and bandwidth changes in terms of structure and dynamics of the sample. There is a focus on recent progress in the analysis of the acoustic ratio, ΔΓ/(−Δf), and numerical methods of modelling. Limitations of the existing approaches for data analysis are discussed. Challenges and possible future developments are formulated in an outlook.
The full article can be accessed here.


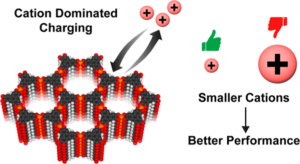

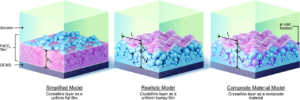 This paper reports the real time monitoring of siderite deposition, on both Au- and Fe-coated surfaces, using the changes in frequency and dissipation of quartz crystal microbalance with dissipation (QCMD). In an iron chloride solution saturated with carbon dioxide, buffered with sodium bicarbonate to pH 6.8, roughly spherical particles of siderite formed within 15 min, which subsequently deposited on the QCMD crystal surface. Imaging of the surface showed a layer formed from particles ca. < 0.5 μm in diameter. Larger particles are clearly deposited on top of the lower layer; these larger particles are >1 μm in diameter. Monitoring of the frequency clearly differentiates the formation of the lower layer from the larger crystals deposited on top at later times. The elastic moduli calculated from QCMD data showed a progressive dissipation increase; the modeling of the solid–liquid interface using a flat approximation resulted in a poor estimation of elastic and storage moduli. Rather, the impedance modeled as a viscoelastic layer in contact with a semi-infinite liquid, where a random bumpy surface with a Gaussian correlator is used, is much more accurate in determining the elastic and storage moduli as losses from the uneven interface are considered. A further step considers that the film is in fact a composite consisting of hard spherical particles of siderite with water in the vacant spaces. This is treated by considering the individual contributions of the phases to the losses measured, thereby further improving the accuracy of the description of the film and the QCMD data. Collectively, this work presents a new framework for the use of QCMD, paired with traditional approaches, to enhance the understanding of crystal deposition and film formation as well as quantify the often evolving mechanical properties.
This paper reports the real time monitoring of siderite deposition, on both Au- and Fe-coated surfaces, using the changes in frequency and dissipation of quartz crystal microbalance with dissipation (QCMD). In an iron chloride solution saturated with carbon dioxide, buffered with sodium bicarbonate to pH 6.8, roughly spherical particles of siderite formed within 15 min, which subsequently deposited on the QCMD crystal surface. Imaging of the surface showed a layer formed from particles ca. < 0.5 μm in diameter. Larger particles are clearly deposited on top of the lower layer; these larger particles are >1 μm in diameter. Monitoring of the frequency clearly differentiates the formation of the lower layer from the larger crystals deposited on top at later times. The elastic moduli calculated from QCMD data showed a progressive dissipation increase; the modeling of the solid–liquid interface using a flat approximation resulted in a poor estimation of elastic and storage moduli. Rather, the impedance modeled as a viscoelastic layer in contact with a semi-infinite liquid, where a random bumpy surface with a Gaussian correlator is used, is much more accurate in determining the elastic and storage moduli as losses from the uneven interface are considered. A further step considers that the film is in fact a composite consisting of hard spherical particles of siderite with water in the vacant spaces. This is treated by considering the individual contributions of the phases to the losses measured, thereby further improving the accuracy of the description of the film and the QCMD data. Collectively, this work presents a new framework for the use of QCMD, paired with traditional approaches, to enhance the understanding of crystal deposition and film formation as well as quantify the often evolving mechanical properties.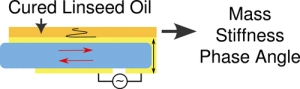 Drying oils such as linseed oil form a polymer network through a complex free-radical polymerization process. We have studied polymerization in this challenging class of polymers using a quartz crystal microbalance (QCM). The QCM is able to measure the evolution of polymer mass and mechanical properties as the oil transitions from a liquid-like to a solid-like state. Measurements using bulk materials and thin films provide information about the initial polymerization phase as well as the evolution of the mass and mechanical properties over the first two years of cure. The temperature-dependent response of the cured linseed oil films was also measured. These results were combined with previously published results obtained from traditional dynamic mechanical analysis to give a unified picture of the properties of these materials across a very broad temperature range.
Drying oils such as linseed oil form a polymer network through a complex free-radical polymerization process. We have studied polymerization in this challenging class of polymers using a quartz crystal microbalance (QCM). The QCM is able to measure the evolution of polymer mass and mechanical properties as the oil transitions from a liquid-like to a solid-like state. Measurements using bulk materials and thin films provide information about the initial polymerization phase as well as the evolution of the mass and mechanical properties over the first two years of cure. The temperature-dependent response of the cured linseed oil films was also measured. These results were combined with previously published results obtained from traditional dynamic mechanical analysis to give a unified picture of the properties of these materials across a very broad temperature range. The single-chain physics of bottlebrush polymers plays a key role in their macroscopic properties. Although efforts have been made to understand the behavior of single isolated bottlebrushes, studies on their behavior in crowded, application-relevant environments have been insufficient due to limitations in characterization techniques. Here, we use single-molecule localization microscopy (SMLM) to study the conformations of individual bottlebrush polymers by direct imaging. Our previous work focused on bottlebrushes in a matrix of linear polymers, where our observations suggested that their behavior was largely influenced by an entropic incompatibility between the bottlebrush side chains and the linear matrix. Instead, here we focus on systems where this effect is reduced: in solvent-swollen polymer materials and in systems entirely composed of bottlebrushes. We measure chain conformations and rigidity using persistence length (lp) as side chain molecular weight (Msc) is varied. Compared to a system of linear polymers, we observe greater flexibility of the backbone in both systems. For bottlebrushes in bottlebrush matrices, we additionally observed a scaling relationship between lp and Msc that more closely follows theoretical predictions. For the more flexible chains in both systems, we reach the edge of our resolution limit and cannot visualize the entire contour of every chain. We bypass this limitation by discussing the aspect ratios of the features within the super-resolution images.
The single-chain physics of bottlebrush polymers plays a key role in their macroscopic properties. Although efforts have been made to understand the behavior of single isolated bottlebrushes, studies on their behavior in crowded, application-relevant environments have been insufficient due to limitations in characterization techniques. Here, we use single-molecule localization microscopy (SMLM) to study the conformations of individual bottlebrush polymers by direct imaging. Our previous work focused on bottlebrushes in a matrix of linear polymers, where our observations suggested that their behavior was largely influenced by an entropic incompatibility between the bottlebrush side chains and the linear matrix. Instead, here we focus on systems where this effect is reduced: in solvent-swollen polymer materials and in systems entirely composed of bottlebrushes. We measure chain conformations and rigidity using persistence length (lp) as side chain molecular weight (Msc) is varied. Compared to a system of linear polymers, we observe greater flexibility of the backbone in both systems. For bottlebrushes in bottlebrush matrices, we additionally observed a scaling relationship between lp and Msc that more closely follows theoretical predictions. For the more flexible chains in both systems, we reach the edge of our resolution limit and cannot visualize the entire contour of every chain. We bypass this limitation by discussing the aspect ratios of the features within the super-resolution images.
