Operando Gravimetric and Energy Loss Analysis of Na3V2(PO4)2F3 Composite Films by Electrochemical Quartz Microbalance with Dissipation Monitoring
Authors: Jeronimo Mirand, Pierre-Louis Taberna, Patrice Simon
Journal: ACS Nano
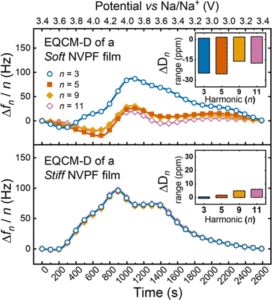 Abstract: The rising demand for energy storage calls for technological advancements to address the growing needs. In this context, sodium-ion (Na-ion) batteries have emerged as a potential complementary technology to lithium-ion batteries (Li-ion). Among other materials, Na3V2(PO4)2F3 (NVPF) is a promising cathode for Na-ion batteries due to its high operating voltage and good energy density. In order to further characterize the (dis)charge behavior of NVPF, the electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D) was employed to track both the frequency and dissipation loss changes at the electrode/electrolyte interface. The electrode composite preparation proved to be crucial for extending the potential window to both Na3V2(PO4)2F3/Na2V2(PO4)2F3 and Na2V2(PO4)2F3/Na1V2(PO4)2F3 domains. Composites prepared with rawNVPF powder (1–20 μm particles) and polyvinylidene fluoride (PVDF) binder (raw-NVPF:PVDF) exhibited large dissipation changes during (dis)charging, attributed to the soft viscoelastic nature of the binder and substantial hydrodynamic interaction caused by the large particles. On the other hand, composites prepared by sieved NVPF particles (<1 μm particles) with sodium carboxymethyl cellulose (NaCMC) binder (sieved-NVPF:NaCMC) showed rigid properties, enabling an extended and more accurate gravimetric analysis. This allowed for the determination of a linear charge-to-mass relationship for the full potential window of NVPF, reflecting the potential independent insertion/deinsertion of bare Na ions (23 g·mol–1). Additionally, reversible dissipative changes were observed for the Na3V2(PO4)2F3/Na2V2(PO4)2F3 transition, with no further dissipative changes observed during the Na2V2(PO4)2F3/Na1V2(PO4)2F3 process
Abstract: The rising demand for energy storage calls for technological advancements to address the growing needs. In this context, sodium-ion (Na-ion) batteries have emerged as a potential complementary technology to lithium-ion batteries (Li-ion). Among other materials, Na3V2(PO4)2F3 (NVPF) is a promising cathode for Na-ion batteries due to its high operating voltage and good energy density. In order to further characterize the (dis)charge behavior of NVPF, the electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D) was employed to track both the frequency and dissipation loss changes at the electrode/electrolyte interface. The electrode composite preparation proved to be crucial for extending the potential window to both Na3V2(PO4)2F3/Na2V2(PO4)2F3 and Na2V2(PO4)2F3/Na1V2(PO4)2F3 domains. Composites prepared with rawNVPF powder (1–20 μm particles) and polyvinylidene fluoride (PVDF) binder (raw-NVPF:PVDF) exhibited large dissipation changes during (dis)charging, attributed to the soft viscoelastic nature of the binder and substantial hydrodynamic interaction caused by the large particles. On the other hand, composites prepared by sieved NVPF particles (<1 μm particles) with sodium carboxymethyl cellulose (NaCMC) binder (sieved-NVPF:NaCMC) showed rigid properties, enabling an extended and more accurate gravimetric analysis. This allowed for the determination of a linear charge-to-mass relationship for the full potential window of NVPF, reflecting the potential independent insertion/deinsertion of bare Na ions (23 g·mol–1). Additionally, reversible dissipative changes were observed for the Na3V2(PO4)2F3/Na2V2(PO4)2F3 transition, with no further dissipative changes observed during the Na2V2(PO4)2F3/Na1V2(PO4)2F3 process


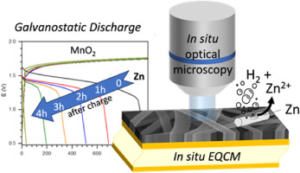 We introduce a novel approach to Zinc-MnO2 battery architecture utilizing a 3D network of carbon nanofibers as both current collector and electrode material, promising enhanced performance and longevity for large-scale energy storage. Employing mild aqueous electrolytes, we address the challenge of managing self-discharge, crucial for short-term energy storage. Advanced coupled characterization techniques, including in-situ EQCM (Electrochemical Quartz Crystal Microbalance) and high-resolution optical microscopy, elucidate self-discharge mechanisms across over multiple length scales. Findings reveal that the self-discharge is mainly at the zinc electrode due to concomitant dissolution of Zinc (corrosion) and HER (Hydrogen Evolution Reaction) phenomena. Interestingly, the corrosion current was estimated irrespective of charging protocol and remains consistent, indicating the independence of zinc corrosion kinetics from the length scale. Finally, the morphology of the zinc layer appears to be critical, suggesting that self-discharge is primarily a chemical process. This innovative design strategy offers the potential for high-performance Zinc-MnO2 batteries with extended cycle life to meet the requirements of large-scale energy storage applications.
We introduce a novel approach to Zinc-MnO2 battery architecture utilizing a 3D network of carbon nanofibers as both current collector and electrode material, promising enhanced performance and longevity for large-scale energy storage. Employing mild aqueous electrolytes, we address the challenge of managing self-discharge, crucial for short-term energy storage. Advanced coupled characterization techniques, including in-situ EQCM (Electrochemical Quartz Crystal Microbalance) and high-resolution optical microscopy, elucidate self-discharge mechanisms across over multiple length scales. Findings reveal that the self-discharge is mainly at the zinc electrode due to concomitant dissolution of Zinc (corrosion) and HER (Hydrogen Evolution Reaction) phenomena. Interestingly, the corrosion current was estimated irrespective of charging protocol and remains consistent, indicating the independence of zinc corrosion kinetics from the length scale. Finally, the morphology of the zinc layer appears to be critical, suggesting that self-discharge is primarily a chemical process. This innovative design strategy offers the potential for high-performance Zinc-MnO2 batteries with extended cycle life to meet the requirements of large-scale energy storage applications.
 Abstract:
Abstract: 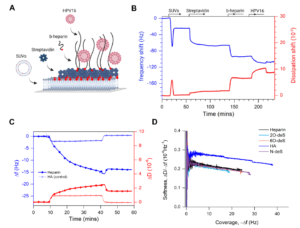 Abstract:
Abstract: 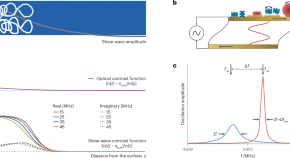 Abstract:
Abstract: