Authors: Avery E. Baumann, Takeshi Yamada, Kanae Ito, Chad R. Snyder, John R. Hoffman, Craig M. Brown, Christopher M. Stafford, and Christopher L. Soles
Journal: Chem. Mater.
Abstract
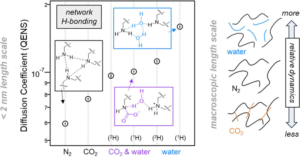 Aminopolymer sorbents are leading candidates for extracting CO2 directly from the atmosphere under ambient conditions. For effective carbon capture, this requires not only that the CO2 actively binds with amine groups of the polymer under low gas concentrations but also that it readily diffuses through the sorbent media to access as many of the amine binding sites as possible. Unfortunately, high reactivity and diffusivity tend to be mutually exclusive properties when it comes to small molecule transport within a polymer, posing a significant materials design challenge. While many reports to date focus on chemical additives or engineering strategies to tackle this trade-off, only a few studies have seriously investigated the underlying chemical and physical properties of the sorbent polymer as a function of its interaction with the relevant sorbate molecules. In this study, we investigate the interplay of polymer-sorbate reactivity and diffusive dynamics of both H2O and CO2 in branched polyethylenimine (PEI) using quasielastic neutron scattering (QENS), infrared spectroscopy, gravimetric uptake, and mechanical dissipation measurements as a function of atmospheric dosing conditions. We uncover an intriguing and previously unreported discrepancy in the diffusive dynamics of PEI dosed with CO2 and H2O vapor at the microscopic and macroscopic length scales. At the macroscopic scale, our mechanical dissipation measurements show that while the exposure to H2O vapor alone always plasticizes the dynamics of PEI, the absorption of CO2, either in the presence of H2O or not, leads to a mechanical stiffening of the PEI. Interestingly, this response differs at the microscopic scale where the diffusive dynamics of the H2O- and/or CO2-dosed samples as quantified by QENS are always enhanced relative to the undosed PEI. This dynamic facilitation is greatest in the presence of H2O vapor alone, consistent with H2O strongly plasticizing the dynamics of PEI. However, the simultaneous exposure to both H2O and CO2 leads to a stiffening of the QENS dynamics at the microscopic scale relative to the hydrated state, signifying local interactions between the CO2 and the polymer. Under these conditions, we also observe a greater amount of CO2 absorbed into the PEI film that is simultaneously exposed to both H2O and CO2 as compared to the film exposed to just CO2, further evidencing a complicated three-way interaction between the H2O, CO2, and PEI. These results are discussed in terms of an absorption process that involves the formation of carbamate ions, the generation of ionic cross-link junctions in the PEI, and changes in the local hydration level of the polymer around the ions. To establish the importance of the carbamate ions in this process, we utilize a methylation reaction to modify the PEI and convert all of the primary and secondary amines into tertiary amines that are incapable of forming carbamates. This considerably diminishes the role of hydrogen bonding in the PEI, enhances the microscopic dynamics of the undosed PEI, and results in diffusive dynamics that do not depend heavily on dosing with H2O and/or CO2. The observations reported here provide insights into the design of next-generation aminopolymer sorbents where both reactivity and diffusive dynamics can be optimized.
Aminopolymer sorbents are leading candidates for extracting CO2 directly from the atmosphere under ambient conditions. For effective carbon capture, this requires not only that the CO2 actively binds with amine groups of the polymer under low gas concentrations but also that it readily diffuses through the sorbent media to access as many of the amine binding sites as possible. Unfortunately, high reactivity and diffusivity tend to be mutually exclusive properties when it comes to small molecule transport within a polymer, posing a significant materials design challenge. While many reports to date focus on chemical additives or engineering strategies to tackle this trade-off, only a few studies have seriously investigated the underlying chemical and physical properties of the sorbent polymer as a function of its interaction with the relevant sorbate molecules. In this study, we investigate the interplay of polymer-sorbate reactivity and diffusive dynamics of both H2O and CO2 in branched polyethylenimine (PEI) using quasielastic neutron scattering (QENS), infrared spectroscopy, gravimetric uptake, and mechanical dissipation measurements as a function of atmospheric dosing conditions. We uncover an intriguing and previously unreported discrepancy in the diffusive dynamics of PEI dosed with CO2 and H2O vapor at the microscopic and macroscopic length scales. At the macroscopic scale, our mechanical dissipation measurements show that while the exposure to H2O vapor alone always plasticizes the dynamics of PEI, the absorption of CO2, either in the presence of H2O or not, leads to a mechanical stiffening of the PEI. Interestingly, this response differs at the microscopic scale where the diffusive dynamics of the H2O- and/or CO2-dosed samples as quantified by QENS are always enhanced relative to the undosed PEI. This dynamic facilitation is greatest in the presence of H2O vapor alone, consistent with H2O strongly plasticizing the dynamics of PEI. However, the simultaneous exposure to both H2O and CO2 leads to a stiffening of the QENS dynamics at the microscopic scale relative to the hydrated state, signifying local interactions between the CO2 and the polymer. Under these conditions, we also observe a greater amount of CO2 absorbed into the PEI film that is simultaneously exposed to both H2O and CO2 as compared to the film exposed to just CO2, further evidencing a complicated three-way interaction between the H2O, CO2, and PEI. These results are discussed in terms of an absorption process that involves the formation of carbamate ions, the generation of ionic cross-link junctions in the PEI, and changes in the local hydration level of the polymer around the ions. To establish the importance of the carbamate ions in this process, we utilize a methylation reaction to modify the PEI and convert all of the primary and secondary amines into tertiary amines that are incapable of forming carbamates. This considerably diminishes the role of hydrogen bonding in the PEI, enhances the microscopic dynamics of the undosed PEI, and results in diffusive dynamics that do not depend heavily on dosing with H2O and/or CO2. The observations reported here provide insights into the design of next-generation aminopolymer sorbents where both reactivity and diffusive dynamics can be optimized.
You can access the full publication here.
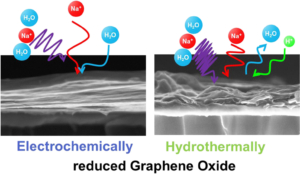 Reduced Graphene Oxide possesses numerous interesting properties, making it one of the most studied materials today. By this way, applications in various fields, including fundamental research, can be found. Nevertheless, the complexity of reduced Graphene Oxide lies in its fabrication process which defines their properties. In this paper, two fabrication methods -electrochemical and hydrothermal reduction of graphene oxide – were compared using physico-chemical and electrogravimetric analysis. Our findings reveal significant morphological differences between the two methods, accompanied by different electrochemical behaviors, when tested in aqueous electrolyte (i.e. 0.5 M Na2SO4). Specifically, electrochemically reduced graphene oxide exclusively involves sodium (whether hydrated or not) in its charge compensation mechanism, whereas hydrothermally reduced graphene oxide also involves proton in sodium sulfate solution.
Reduced Graphene Oxide possesses numerous interesting properties, making it one of the most studied materials today. By this way, applications in various fields, including fundamental research, can be found. Nevertheless, the complexity of reduced Graphene Oxide lies in its fabrication process which defines their properties. In this paper, two fabrication methods -electrochemical and hydrothermal reduction of graphene oxide – were compared using physico-chemical and electrogravimetric analysis. Our findings reveal significant morphological differences between the two methods, accompanied by different electrochemical behaviors, when tested in aqueous electrolyte (i.e. 0.5 M Na2SO4). Specifically, electrochemically reduced graphene oxide exclusively involves sodium (whether hydrated or not) in its charge compensation mechanism, whereas hydrothermally reduced graphene oxide also involves proton in sodium sulfate solution.

 Aminopolymer sorbents are leading candidates for extracting CO2 directly from the atmosphere under ambient conditions. For effective carbon capture, this requires not only that the CO2 actively binds with amine groups of the polymer under low gas concentrations but also that it readily diffuses through the sorbent media to access as many of the amine binding sites as possible. Unfortunately, high reactivity and diffusivity tend to be mutually exclusive properties when it comes to small molecule transport within a polymer, posing a significant materials design challenge. While many reports to date focus on chemical additives or engineering strategies to tackle this trade-off, only a few studies have seriously investigated the underlying chemical and physical properties of the sorbent polymer as a function of its interaction with the relevant sorbate molecules. In this study, we investigate the interplay of polymer-sorbate reactivity and diffusive dynamics of both H2O and CO2 in branched polyethylenimine (PEI) using quasielastic neutron scattering (QENS), infrared spectroscopy, gravimetric uptake, and mechanical dissipation measurements as a function of atmospheric dosing conditions. We uncover an intriguing and previously unreported discrepancy in the diffusive dynamics of PEI dosed with CO2 and H2O vapor at the microscopic and macroscopic length scales. At the macroscopic scale, our mechanical dissipation measurements show that while the exposure to H2O vapor alone always plasticizes the dynamics of PEI, the absorption of CO2, either in the presence of H2O or not, leads to a mechanical stiffening of the PEI. Interestingly, this response differs at the microscopic scale where the diffusive dynamics of the H2O- and/or CO2-dosed samples as quantified by QENS are always enhanced relative to the undosed PEI. This dynamic facilitation is greatest in the presence of H2O vapor alone, consistent with H2O strongly plasticizing the dynamics of PEI. However, the simultaneous exposure to both H2O and CO2 leads to a stiffening of the QENS dynamics at the microscopic scale relative to the hydrated state, signifying local interactions between the CO2 and the polymer. Under these conditions, we also observe a greater amount of CO2 absorbed into the PEI film that is simultaneously exposed to both H2O and CO2 as compared to the film exposed to just CO2, further evidencing a complicated three-way interaction between the H2O, CO2, and PEI. These results are discussed in terms of an absorption process that involves the formation of carbamate ions, the generation of ionic cross-link junctions in the PEI, and changes in the local hydration level of the polymer around the ions. To establish the importance of the carbamate ions in this process, we utilize a methylation reaction to modify the PEI and convert all of the primary and secondary amines into tertiary amines that are incapable of forming carbamates. This considerably diminishes the role of hydrogen bonding in the PEI, enhances the microscopic dynamics of the undosed PEI, and results in diffusive dynamics that do not depend heavily on dosing with H2O and/or CO2. The observations reported here provide insights into the design of next-generation aminopolymer sorbents where both reactivity and diffusive dynamics can be optimized.
Aminopolymer sorbents are leading candidates for extracting CO2 directly from the atmosphere under ambient conditions. For effective carbon capture, this requires not only that the CO2 actively binds with amine groups of the polymer under low gas concentrations but also that it readily diffuses through the sorbent media to access as many of the amine binding sites as possible. Unfortunately, high reactivity and diffusivity tend to be mutually exclusive properties when it comes to small molecule transport within a polymer, posing a significant materials design challenge. While many reports to date focus on chemical additives or engineering strategies to tackle this trade-off, only a few studies have seriously investigated the underlying chemical and physical properties of the sorbent polymer as a function of its interaction with the relevant sorbate molecules. In this study, we investigate the interplay of polymer-sorbate reactivity and diffusive dynamics of both H2O and CO2 in branched polyethylenimine (PEI) using quasielastic neutron scattering (QENS), infrared spectroscopy, gravimetric uptake, and mechanical dissipation measurements as a function of atmospheric dosing conditions. We uncover an intriguing and previously unreported discrepancy in the diffusive dynamics of PEI dosed with CO2 and H2O vapor at the microscopic and macroscopic length scales. At the macroscopic scale, our mechanical dissipation measurements show that while the exposure to H2O vapor alone always plasticizes the dynamics of PEI, the absorption of CO2, either in the presence of H2O or not, leads to a mechanical stiffening of the PEI. Interestingly, this response differs at the microscopic scale where the diffusive dynamics of the H2O- and/or CO2-dosed samples as quantified by QENS are always enhanced relative to the undosed PEI. This dynamic facilitation is greatest in the presence of H2O vapor alone, consistent with H2O strongly plasticizing the dynamics of PEI. However, the simultaneous exposure to both H2O and CO2 leads to a stiffening of the QENS dynamics at the microscopic scale relative to the hydrated state, signifying local interactions between the CO2 and the polymer. Under these conditions, we also observe a greater amount of CO2 absorbed into the PEI film that is simultaneously exposed to both H2O and CO2 as compared to the film exposed to just CO2, further evidencing a complicated three-way interaction between the H2O, CO2, and PEI. These results are discussed in terms of an absorption process that involves the formation of carbamate ions, the generation of ionic cross-link junctions in the PEI, and changes in the local hydration level of the polymer around the ions. To establish the importance of the carbamate ions in this process, we utilize a methylation reaction to modify the PEI and convert all of the primary and secondary amines into tertiary amines that are incapable of forming carbamates. This considerably diminishes the role of hydrogen bonding in the PEI, enhances the microscopic dynamics of the undosed PEI, and results in diffusive dynamics that do not depend heavily on dosing with H2O and/or CO2. The observations reported here provide insights into the design of next-generation aminopolymer sorbents where both reactivity and diffusive dynamics can be optimized.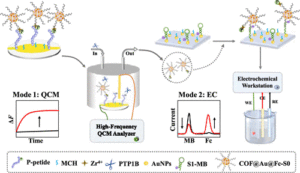 The abnormal expression of protein tyrosine phosphatase 1B (PTP1B) is highly related to several serious human diseases. Therefore, an accurate PTP1B activity assay is beneficial to the diagnosis and treatment of these diseases. In this study, a dual-mode biosensing platform that enabled the sensitive and accurate assay of PTP1B activity was constructed based on the high-frequency (100 MHz) quartz crystal microbalance (QCM) and dual-signaling electrochemical (EC) ratiometric strategy. Covalent–organic framework@gold nanoparticles@ferrocene@single-strand DNA (COF@Au@Fc-S0) was introduced onto the QCM Au chip via the chelation between Zr4+ and phosphate groups (phosphate group of the phosphopeptide (P-peptide) on the QCM Au chip and the phosphate group of thiol-labeled single-stranded DNA (S0) on COF@Au@Fc-S0) and used as a signal reporter. When PTP1B was present, the dephosphorylation of the P-peptide led to the release of COF@Au@Fc-S0 from the QCM Au chip, resulting in an increase in the frequency of the QCM. Meanwhile, the released COF@Au@Fc-S0 hybridized with thiol/methylene blue (MB)-labeled hairpin DNA (S1-MB) on the Au NPs-modified indium–tin oxide (ITO) electrode. This caused MB to be far away from the electrode surface and Fc to be close to the electrode, leading to a decrease in the oxidation peak current of MB and an increase in the oxidation peak current of Fc. Thus, PTP1B-induced dephosphorylation of the P-peptide was monitored in real time by QCM, and PTP1B activity was detected sensitively and reliably using this innovative QCM-EC dual-mode sensing platform with an ultralow detection limit. This platform is anticipated to serve as a robust tool for the analysis of protein phosphatase activity and the discovery of drugs targeting protein phosphatase.
The abnormal expression of protein tyrosine phosphatase 1B (PTP1B) is highly related to several serious human diseases. Therefore, an accurate PTP1B activity assay is beneficial to the diagnosis and treatment of these diseases. In this study, a dual-mode biosensing platform that enabled the sensitive and accurate assay of PTP1B activity was constructed based on the high-frequency (100 MHz) quartz crystal microbalance (QCM) and dual-signaling electrochemical (EC) ratiometric strategy. Covalent–organic framework@gold nanoparticles@ferrocene@single-strand DNA (COF@Au@Fc-S0) was introduced onto the QCM Au chip via the chelation between Zr4+ and phosphate groups (phosphate group of the phosphopeptide (P-peptide) on the QCM Au chip and the phosphate group of thiol-labeled single-stranded DNA (S0) on COF@Au@Fc-S0) and used as a signal reporter. When PTP1B was present, the dephosphorylation of the P-peptide led to the release of COF@Au@Fc-S0 from the QCM Au chip, resulting in an increase in the frequency of the QCM. Meanwhile, the released COF@Au@Fc-S0 hybridized with thiol/methylene blue (MB)-labeled hairpin DNA (S1-MB) on the Au NPs-modified indium–tin oxide (ITO) electrode. This caused MB to be far away from the electrode surface and Fc to be close to the electrode, leading to a decrease in the oxidation peak current of MB and an increase in the oxidation peak current of Fc. Thus, PTP1B-induced dephosphorylation of the P-peptide was monitored in real time by QCM, and PTP1B activity was detected sensitively and reliably using this innovative QCM-EC dual-mode sensing platform with an ultralow detection limit. This platform is anticipated to serve as a robust tool for the analysis of protein phosphatase activity and the discovery of drugs targeting protein phosphatase.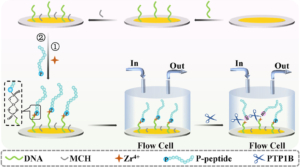 The misregulation of protein phosphatases is a key factor in the development of many human diseases, notably cancers. Here, based on a 100 MHz quartz crystal microbalance (QCM) biosensing platform, the dephosphorylation process of phosphopeptide (P-peptide) caused by protein tyrosine phosphatase 1B (PTP1B) was monitored in real time for the first time and PTP1B activity was assayed rapidly and sensitively. The QCM chip, coated with a gold (Au) film, was used to immobilized thiol-labeled single-stranded 5′-phosphate-DNAs (P-DNA) through Au–S bond. The P-peptide, specific to PTP1B, was then connected to the P-DNA via chelation between Zr4+ and phosphate groups. When PTP1B was injected into the QCM flow cell where the P-peptide/Zr4+/MCH/P-DNA/Au chip was placed, the P-peptide was dephosphorylated and released from the Au chip surface, resulting in an increase in the frequency of the QCM Au chip. This allowed the real-time monitoring of the P-peptide dephosphorylation process and sensitive detection of PTP1B activity within 6 min with a linear detection range of 0.01–100 pM and a detection limit of 0.008 pM. In addition, the maximum inhibitory ratios of inhibitors were evaluated using this proposed 100 MHz QCM biosensor. The developed 100 MHz QCM biosensing platform shows immense potential for early diagnosis of diseases related to protein phosphatases and the development of drugs targeting protein phosphatases.
The misregulation of protein phosphatases is a key factor in the development of many human diseases, notably cancers. Here, based on a 100 MHz quartz crystal microbalance (QCM) biosensing platform, the dephosphorylation process of phosphopeptide (P-peptide) caused by protein tyrosine phosphatase 1B (PTP1B) was monitored in real time for the first time and PTP1B activity was assayed rapidly and sensitively. The QCM chip, coated with a gold (Au) film, was used to immobilized thiol-labeled single-stranded 5′-phosphate-DNAs (P-DNA) through Au–S bond. The P-peptide, specific to PTP1B, was then connected to the P-DNA via chelation between Zr4+ and phosphate groups. When PTP1B was injected into the QCM flow cell where the P-peptide/Zr4+/MCH/P-DNA/Au chip was placed, the P-peptide was dephosphorylated and released from the Au chip surface, resulting in an increase in the frequency of the QCM Au chip. This allowed the real-time monitoring of the P-peptide dephosphorylation process and sensitive detection of PTP1B activity within 6 min with a linear detection range of 0.01–100 pM and a detection limit of 0.008 pM. In addition, the maximum inhibitory ratios of inhibitors were evaluated using this proposed 100 MHz QCM biosensor. The developed 100 MHz QCM biosensing platform shows immense potential for early diagnosis of diseases related to protein phosphatases and the development of drugs targeting protein phosphatases.
