Aerosol Jet Printing of the Ultramicroporous Calcium Squarate Metal–Organic Framework
Authors: Dmitry E. Kravchenko, Aleksander Matavž, Víctor Rubio-Giménez, Hanne Vanduffel, Margot Verstreken, Rob Ameloot
Journal: Chem. Mater. (2022)
Abstract
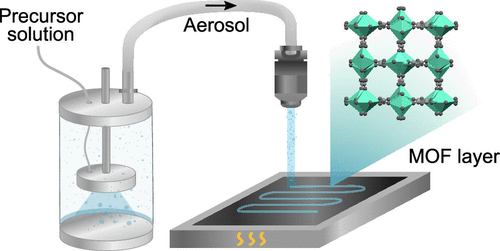
Efficient methods to deposit thin layers of metal–organic frameworks (MOFs) are needed to integrate these microporous materials into microelectronics, sensing devices, and membranes. Herein, we report for the first time the direct aerosol jet printing of a MOF material. The ultramicroporous MOF [Ca(C4O4) (H2O)] (UTSA-280) was deposited from an aqueous precursor solution. In addition to blanket coatings, aerosol jet printing provides direct access to patterned coatings with a resolution of 100 μm via a digital, maskless approach. Moreover, by enabling spatial control over the layer thickness via the number of passes of the nozzle, this direct-write approach presents a more accessible alternative to advanced patterning techniques such as grayscale lithography.
You may read the full paper here.
Aerosol Jet Printing of the Ultramicroporous Calcium Squarate Metal–Organic Framework