Operando Tracking of Resistance, Thickness, and Mass of Ti3C2Tx MXene in Water-in-Salt Electrolyte
Authors: Audrey Perju, Danzhen Zhang, Ruocun John Wang, Pierre-Louis Taberna, Yury Gogotsi, Patrice Simon
Journal: Adv. Energy Mater.
 Abstract: MXenes are among the fastest-growing families of 2D materials, promising for high-rate, high-energy energy storage applications due to their high electronic and ionic conductivity, large surface area, and reversible surface redox ability. The Ti3C2Tx MXene shows a capacitive charge storage mechanism in diluted aqueous LiCl electrolyte while achieving abnormal redox-like features in the water-in-salt LiCl electrolyte. Herein, various operando techniques are used to investigate changes in resistance, mass, and electrode thickness of Ti3C2Tx during cycling in salt-in-water and water-in-salt LiCl electrolytes. Significant resistance variations due to interlayer space changes are recorded in the water-in-salt LiCl electrolyte. In both electrolytes, conductivity variations attributed to charge carrier density changes or varied inter-sheet electron hopping barriers are detected in the capacitive areas, where no thickness variations are observed. Overall, combining those operando techniques enhances the understanding of charge storage mechanisms and facilitates the development of MXene-based energy storage devices.
Abstract: MXenes are among the fastest-growing families of 2D materials, promising for high-rate, high-energy energy storage applications due to their high electronic and ionic conductivity, large surface area, and reversible surface redox ability. The Ti3C2Tx MXene shows a capacitive charge storage mechanism in diluted aqueous LiCl electrolyte while achieving abnormal redox-like features in the water-in-salt LiCl electrolyte. Herein, various operando techniques are used to investigate changes in resistance, mass, and electrode thickness of Ti3C2Tx during cycling in salt-in-water and water-in-salt LiCl electrolytes. Significant resistance variations due to interlayer space changes are recorded in the water-in-salt LiCl electrolyte. In both electrolytes, conductivity variations attributed to charge carrier density changes or varied inter-sheet electron hopping barriers are detected in the capacitive areas, where no thickness variations are observed. Overall, combining those operando techniques enhances the understanding of charge storage mechanisms and facilitates the development of MXene-based energy storage devices.


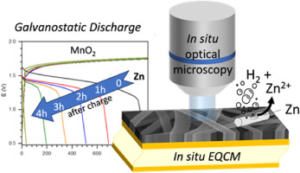 We introduce a novel approach to Zinc-MnO2 battery architecture utilizing a 3D network of carbon nanofibers as both current collector and electrode material, promising enhanced performance and longevity for large-scale energy storage. Employing mild aqueous electrolytes, we address the challenge of managing self-discharge, crucial for short-term energy storage. Advanced coupled characterization techniques, including in-situ EQCM (Electrochemical Quartz Crystal Microbalance) and high-resolution optical microscopy, elucidate self-discharge mechanisms across over multiple length scales. Findings reveal that the self-discharge is mainly at the zinc electrode due to concomitant dissolution of Zinc (corrosion) and HER (Hydrogen Evolution Reaction) phenomena. Interestingly, the corrosion current was estimated irrespective of charging protocol and remains consistent, indicating the independence of zinc corrosion kinetics from the length scale. Finally, the morphology of the zinc layer appears to be critical, suggesting that self-discharge is primarily a chemical process. This innovative design strategy offers the potential for high-performance Zinc-MnO2 batteries with extended cycle life to meet the requirements of large-scale energy storage applications.
We introduce a novel approach to Zinc-MnO2 battery architecture utilizing a 3D network of carbon nanofibers as both current collector and electrode material, promising enhanced performance and longevity for large-scale energy storage. Employing mild aqueous electrolytes, we address the challenge of managing self-discharge, crucial for short-term energy storage. Advanced coupled characterization techniques, including in-situ EQCM (Electrochemical Quartz Crystal Microbalance) and high-resolution optical microscopy, elucidate self-discharge mechanisms across over multiple length scales. Findings reveal that the self-discharge is mainly at the zinc electrode due to concomitant dissolution of Zinc (corrosion) and HER (Hydrogen Evolution Reaction) phenomena. Interestingly, the corrosion current was estimated irrespective of charging protocol and remains consistent, indicating the independence of zinc corrosion kinetics from the length scale. Finally, the morphology of the zinc layer appears to be critical, suggesting that self-discharge is primarily a chemical process. This innovative design strategy offers the potential for high-performance Zinc-MnO2 batteries with extended cycle life to meet the requirements of large-scale energy storage applications.