Unlocking self-discharge: Unveiling the mysteries of electrode-free Zn-MnO2 batteries with advanced in situ techniques in mild acid aqueous electrolytes
Authors: Arvinder Singh, Lamia Ouassi, Keho Allemang, Jean-François Lemineur, Ozlem Sel, Frédéric Kanoufi, Christel Laberty-Robert
Journal: Journal of Power Sources 2025
Abstract: 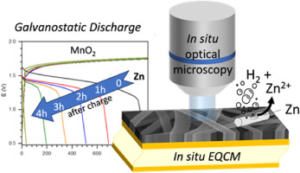 We introduce a novel approach to Zinc-MnO2 battery architecture utilizing a 3D network of carbon nanofibers as both current collector and electrode material, promising enhanced performance and longevity for large-scale energy storage. Employing mild aqueous electrolytes, we address the challenge of managing self-discharge, crucial for short-term energy storage. Advanced coupled characterization techniques, including in-situ EQCM (Electrochemical Quartz Crystal Microbalance) and high-resolution optical microscopy, elucidate self-discharge mechanisms across over multiple length scales. Findings reveal that the self-discharge is mainly at the zinc electrode due to concomitant dissolution of Zinc (corrosion) and HER (Hydrogen Evolution Reaction) phenomena. Interestingly, the corrosion current was estimated irrespective of charging protocol and remains consistent, indicating the independence of zinc corrosion kinetics from the length scale. Finally, the morphology of the zinc layer appears to be critical, suggesting that self-discharge is primarily a chemical process. This innovative design strategy offers the potential for high-performance Zinc-MnO2 batteries with extended cycle life to meet the requirements of large-scale energy storage applications.
We introduce a novel approach to Zinc-MnO2 battery architecture utilizing a 3D network of carbon nanofibers as both current collector and electrode material, promising enhanced performance and longevity for large-scale energy storage. Employing mild aqueous electrolytes, we address the challenge of managing self-discharge, crucial for short-term energy storage. Advanced coupled characterization techniques, including in-situ EQCM (Electrochemical Quartz Crystal Microbalance) and high-resolution optical microscopy, elucidate self-discharge mechanisms across over multiple length scales. Findings reveal that the self-discharge is mainly at the zinc electrode due to concomitant dissolution of Zinc (corrosion) and HER (Hydrogen Evolution Reaction) phenomena. Interestingly, the corrosion current was estimated irrespective of charging protocol and remains consistent, indicating the independence of zinc corrosion kinetics from the length scale. Finally, the morphology of the zinc layer appears to be critical, suggesting that self-discharge is primarily a chemical process. This innovative design strategy offers the potential for high-performance Zinc-MnO2 batteries with extended cycle life to meet the requirements of large-scale energy storage applications.




