Operando Gravimetric and Energy Loss Analysis of Na3V2(PO4)2F3 Composite Films by Electrochemical Quartz Microbalance with Dissipation Monitoring
Authors: Jeronimo Mirand, Pierre-Louis Taberna, Patrice Simon
Journal: ACS Nano
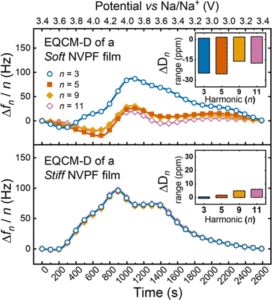 Abstract: The rising demand for energy storage calls for technological advancements to address the growing needs. In this context, sodium-ion (Na-ion) batteries have emerged as a potential complementary technology to lithium-ion batteries (Li-ion). Among other materials, Na3V2(PO4)2F3 (NVPF) is a promising cathode for Na-ion batteries due to its high operating voltage and good energy density. In order to further characterize the (dis)charge behavior of NVPF, the electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D) was employed to track both the frequency and dissipation loss changes at the electrode/electrolyte interface. The electrode composite preparation proved to be crucial for extending the potential window to both Na3V2(PO4)2F3/Na2V2(PO4)2F3 and Na2V2(PO4)2F3/Na1V2(PO4)2F3 domains. Composites prepared with rawNVPF powder (1–20 μm particles) and polyvinylidene fluoride (PVDF) binder (raw-NVPF:PVDF) exhibited large dissipation changes during (dis)charging, attributed to the soft viscoelastic nature of the binder and substantial hydrodynamic interaction caused by the large particles. On the other hand, composites prepared by sieved NVPF particles (<1 μm particles) with sodium carboxymethyl cellulose (NaCMC) binder (sieved-NVPF:NaCMC) showed rigid properties, enabling an extended and more accurate gravimetric analysis. This allowed for the determination of a linear charge-to-mass relationship for the full potential window of NVPF, reflecting the potential independent insertion/deinsertion of bare Na ions (23 g·mol–1). Additionally, reversible dissipative changes were observed for the Na3V2(PO4)2F3/Na2V2(PO4)2F3 transition, with no further dissipative changes observed during the Na2V2(PO4)2F3/Na1V2(PO4)2F3 process
Abstract: The rising demand for energy storage calls for technological advancements to address the growing needs. In this context, sodium-ion (Na-ion) batteries have emerged as a potential complementary technology to lithium-ion batteries (Li-ion). Among other materials, Na3V2(PO4)2F3 (NVPF) is a promising cathode for Na-ion batteries due to its high operating voltage and good energy density. In order to further characterize the (dis)charge behavior of NVPF, the electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D) was employed to track both the frequency and dissipation loss changes at the electrode/electrolyte interface. The electrode composite preparation proved to be crucial for extending the potential window to both Na3V2(PO4)2F3/Na2V2(PO4)2F3 and Na2V2(PO4)2F3/Na1V2(PO4)2F3 domains. Composites prepared with rawNVPF powder (1–20 μm particles) and polyvinylidene fluoride (PVDF) binder (raw-NVPF:PVDF) exhibited large dissipation changes during (dis)charging, attributed to the soft viscoelastic nature of the binder and substantial hydrodynamic interaction caused by the large particles. On the other hand, composites prepared by sieved NVPF particles (<1 μm particles) with sodium carboxymethyl cellulose (NaCMC) binder (sieved-NVPF:NaCMC) showed rigid properties, enabling an extended and more accurate gravimetric analysis. This allowed for the determination of a linear charge-to-mass relationship for the full potential window of NVPF, reflecting the potential independent insertion/deinsertion of bare Na ions (23 g·mol–1). Additionally, reversible dissipative changes were observed for the Na3V2(PO4)2F3/Na2V2(PO4)2F3 transition, with no further dissipative changes observed during the Na2V2(PO4)2F3/Na1V2(PO4)2F3 process



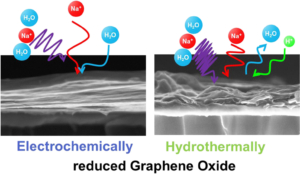 Reduced Graphene Oxide possesses numerous interesting properties, making it one of the most studied materials today. By this way, applications in various fields, including fundamental research, can be found. Nevertheless, the complexity of reduced Graphene Oxide lies in its fabrication process which defines their properties. In this paper, two fabrication methods -electrochemical and hydrothermal reduction of graphene oxide – were compared using physico-chemical and electrogravimetric analysis. Our findings reveal significant morphological differences between the two methods, accompanied by different electrochemical behaviors, when tested in aqueous electrolyte (i.e. 0.5 M Na2SO4). Specifically, electrochemically reduced graphene oxide exclusively involves sodium (whether hydrated or not) in its charge compensation mechanism, whereas hydrothermally reduced graphene oxide also involves proton in sodium sulfate solution.
Reduced Graphene Oxide possesses numerous interesting properties, making it one of the most studied materials today. By this way, applications in various fields, including fundamental research, can be found. Nevertheless, the complexity of reduced Graphene Oxide lies in its fabrication process which defines their properties. In this paper, two fabrication methods -electrochemical and hydrothermal reduction of graphene oxide – were compared using physico-chemical and electrogravimetric analysis. Our findings reveal significant morphological differences between the two methods, accompanied by different electrochemical behaviors, when tested in aqueous electrolyte (i.e. 0.5 M Na2SO4). Specifically, electrochemically reduced graphene oxide exclusively involves sodium (whether hydrated or not) in its charge compensation mechanism, whereas hydrothermally reduced graphene oxide also involves proton in sodium sulfate solution.