The role of humidity in enhancing CO2 capture efficiency in polyethyleneimine thin films
Authors: John R. Hoffman, Avery E. Baumann, Christopher M. Stafford
Journal: Chemical Engineering Journal
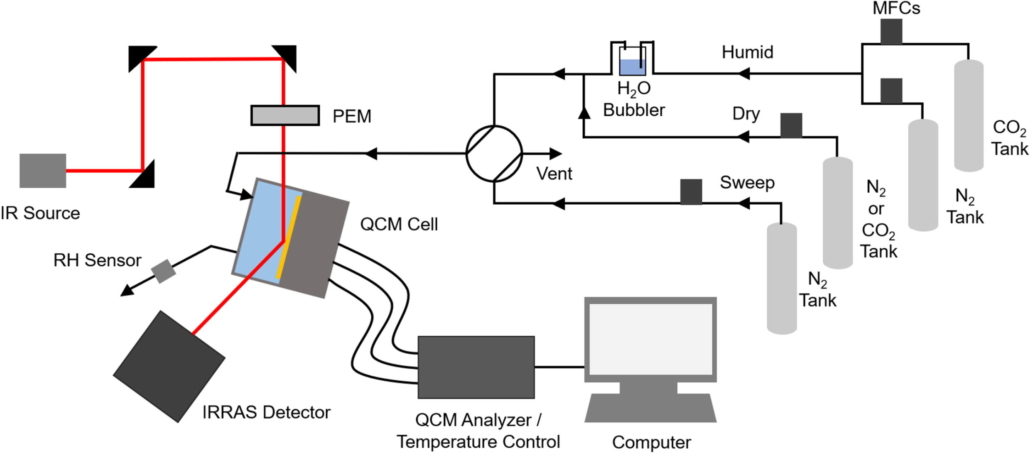 Abstract: Amine impregnated sorbents have been extensively studied for direct air capture (DAC) of CO2 in both dry and humid conditions. In a dry environment, CO2 capture follows a carbamate formation mechanism. Amine efficiency can be improved by allowing more amine sites to participate in the reaction. Introducing water vapor helps break up internal hydrogen bonding within the amine-based polymer, which increases both the polymer mobility and accessibility of amine sites. In this work, we evaluate the influence of humidity on the CO2 capture in polyethyleneimine (PEI) thin films using tandem quartz crystal microbalance (QCM) and polarization modulation infrared reflection–absorption spectroscopy (PM-IRRAS). We show that tandem QCM/PM-IRRAS enables more accurate CO2 uptake measurements, as the combined techniques can separate individual mass changes due to CO2 and H2O sorption. CO2 adsorption capacity and amine efficiency were evaluated for a 10 nm and 100 nm film at varied temperatures, humidities, and CO2 concentrations. We find that water sorption greatly enhances CO2 uptake when the capture is limited by diffusional resistance (at higher CO2 concentration and in 100 nm films) but has less influence in conditions where CO2 availability is limiting uptake (at lower CO2 concentration and in 10 nm films). Thus, we argue that humidity does improve capture, but not at all conditions. Our approach enhances the understanding of H2O-assisted sorption of CO2 in PEI across a range of conditions while also presenting a measurement strategy to apply to, and hopefully optimize, component uptake in materials of interest to the CO2 capture community.
Abstract: Amine impregnated sorbents have been extensively studied for direct air capture (DAC) of CO2 in both dry and humid conditions. In a dry environment, CO2 capture follows a carbamate formation mechanism. Amine efficiency can be improved by allowing more amine sites to participate in the reaction. Introducing water vapor helps break up internal hydrogen bonding within the amine-based polymer, which increases both the polymer mobility and accessibility of amine sites. In this work, we evaluate the influence of humidity on the CO2 capture in polyethyleneimine (PEI) thin films using tandem quartz crystal microbalance (QCM) and polarization modulation infrared reflection–absorption spectroscopy (PM-IRRAS). We show that tandem QCM/PM-IRRAS enables more accurate CO2 uptake measurements, as the combined techniques can separate individual mass changes due to CO2 and H2O sorption. CO2 adsorption capacity and amine efficiency were evaluated for a 10 nm and 100 nm film at varied temperatures, humidities, and CO2 concentrations. We find that water sorption greatly enhances CO2 uptake when the capture is limited by diffusional resistance (at higher CO2 concentration and in 100 nm films) but has less influence in conditions where CO2 availability is limiting uptake (at lower CO2 concentration and in 10 nm films). Thus, we argue that humidity does improve capture, but not at all conditions. Our approach enhances the understanding of H2O-assisted sorption of CO2 in PEI across a range of conditions while also presenting a measurement strategy to apply to, and hopefully optimize, component uptake in materials of interest to the CO2 capture community.