Matrix Metalloproteinase-9 Mediates Endothelial Glycocalyx Degradation and Correlates with Severity of Hemorrhagic Fever with Renal Syndrome
Authors: Chloé Jacquet. Rasmus Gustafsson, Ankit Kumar Patel, Magnus Hansson, Gregory Rankin, Fouzia Bano, Julia Wigren Byström, Anders Blomberg, Johan Rasmuson, Simon Satchell, Therese Thunberg, Clas Ahlm, Marta Bally, Anne-Marie Fors Connolly
Journal: medRxiv Preprint
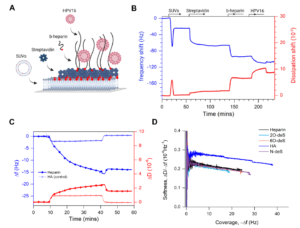
Abstract: Hemorrhagic fever with renal syndrome (HFRS) caused by Puumala virus (PUUV) leads to vascular dysfunction contributing to acute kidney injury and pulmonary complications. The endothelial glycocalyx (eGLX) is crucial for vascular integrity, and its degradation may exacerbate disease severity. In this study, we examined the association between eGLX degradation and renal and pulmonary dysfunction in 44 patients with laboratory-confirmed PUUV infection. We measured plasma levels of eGLX degradation markers—syndecan-1, heparan sulfate, soluble thrombomodulin, and albumin— and found that these correlated with severe acute kidney injury and the need for oxygen therapy. In vitro experiments showed that matrix metalloproteinase-9 (MMP-9) and heparanase can degrade eGLX components, but albumin at physiological concentrations can mitigate this degradation and protect endothelial barrier function. These findings indicate that eGLX degradation contributes to HFRS pathogenesis and suggest that targeting the eGLX could be a therapeutic strategy to improve patient outcomes.


 Abstract:
Abstract: 
