Interface Properties of 2D Graphene–Polydopamine Composite Electrodes in Protic Ionic Liquid-Based Electrolytes Explored by Advanced Electrogravimetry
Authors: Adnane Bouzina, Hubert Perrot, Catherine Debiemme-Chouvy, and Ozlem Sel
Journal: ACS Appl. Energy Mater. (2022)
Abstract
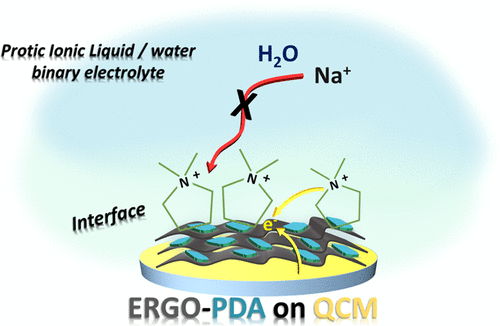
A fundamental understanding of the processes occurring at the electrode/electrolyte interfaces is of paramount importance to enhance the performance of energy storage devices. Addressing this issue requires suitable characterization tools, due to the complex nature of such interfaces. By means of electrochemical quartz crystal microbalance (EQCM) and its advanced mode, the so-called ac-electrogravimetry, herein, we report on the interfacial properties of two-dimensional (2D) graphene–polydopamine (ERGO-PDA) composite electrodes in diverse electrolyte compositions including a protic ionic liquid (PIL), pyrrolidinium hydrogen sulfate [Pyr+][HSO4–]. We have performed a comparative study in a [Pyr+][HSO4–]–water binary mixture in the absence and presence of Na2SO4 and compared it with the interfacial behavior of ERGO-PDA in a 0.5 M Na2SO4 (pH = 2) pristine electrolyte. Our EQCM and ac-electrogravimetric analyses reveal that the [Pyr+] ions, due to their chaotropic nature, inhibit the approach of kosmotropic Na+ ions and water molecules to the interface, suppressing the contribution of electrodragged water molecules, substantially observed in the case of pristine aqueous electrolyte. Despite the dissimilarity of the charge compensation process occurring in the presence of [Pyr+][HSO4–], the ERGO-PDA electrode is able to maintain similar cycling stability (99% for 10,000 cycles at 1000 mV·s–1) and specific capacitance values (325 F·cm–3) compared with the pristine aqueous electrolyte, with the advantage of superior energy density (16.3 versus 8.7 mWh·cm–3) due to a noticeably enlarged potential window in [Pyr+][HSO4–]–water binary mixtures.
You may read the full paper here.
Interface Properties of 2D Graphene–Polydopamine Composite Electrodes in Protic Ionic Liquid-Based Electrolytes Explored by Advanced Electrogravimetry