Measuring the Influence of CO2 and Water Vapor on the Dynamics in Polyethylenimine To Understand the Direct Air Capture of CO2 from the Environment
Authors: Avery E. Baumann, Takeshi Yamada, Kanae Ito, Chad R. Snyder, John R. Hoffman, Craig M. Brown, Christopher M. Stafford, and Christopher L. Soles
Journal: Chem. Mater.
Abstract
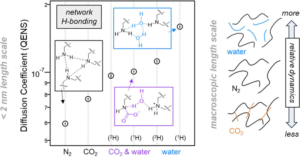 Aminopolymer sorbents are leading candidates for extracting CO2 directly from the atmosphere under ambient conditions. For effective carbon capture, this requires not only that the CO2 actively binds with amine groups of the polymer under low gas concentrations but also that it readily diffuses through the sorbent media to access as many of the amine binding sites as possible. Unfortunately, high reactivity and diffusivity tend to be mutually exclusive properties when it comes to small molecule transport within a polymer, posing a significant materials design challenge. While many reports to date focus on chemical additives or engineering strategies to tackle this trade-off, only a few studies have seriously investigated the underlying chemical and physical properties of the sorbent polymer as a function of its interaction with the relevant sorbate molecules. In this study, we investigate the interplay of polymer-sorbate reactivity and diffusive dynamics of both H2O and CO2 in branched polyethylenimine (PEI) using quasielastic neutron scattering (QENS), infrared spectroscopy, gravimetric uptake, and mechanical dissipation measurements as a function of atmospheric dosing conditions. We uncover an intriguing and previously unreported discrepancy in the diffusive dynamics of PEI dosed with CO2 and H2O vapor at the microscopic and macroscopic length scales. At the macroscopic scale, our mechanical dissipation measurements show that while the exposure to H2O vapor alone always plasticizes the dynamics of PEI, the absorption of CO2, either in the presence of H2O or not, leads to a mechanical stiffening of the PEI. Interestingly, this response differs at the microscopic scale where the diffusive dynamics of the H2O- and/or CO2-dosed samples as quantified by QENS are always enhanced relative to the undosed PEI. This dynamic facilitation is greatest in the presence of H2O vapor alone, consistent with H2O strongly plasticizing the dynamics of PEI. However, the simultaneous exposure to both H2O and CO2 leads to a stiffening of the QENS dynamics at the microscopic scale relative to the hydrated state, signifying local interactions between the CO2 and the polymer. Under these conditions, we also observe a greater amount of CO2 absorbed into the PEI film that is simultaneously exposed to both H2O and CO2 as compared to the film exposed to just CO2, further evidencing a complicated three-way interaction between the H2O, CO2, and PEI. These results are discussed in terms of an absorption process that involves the formation of carbamate ions, the generation of ionic cross-link junctions in the PEI, and changes in the local hydration level of the polymer around the ions. To establish the importance of the carbamate ions in this process, we utilize a methylation reaction to modify the PEI and convert all of the primary and secondary amines into tertiary amines that are incapable of forming carbamates. This considerably diminishes the role of hydrogen bonding in the PEI, enhances the microscopic dynamics of the undosed PEI, and results in diffusive dynamics that do not depend heavily on dosing with H2O and/or CO2. The observations reported here provide insights into the design of next-generation aminopolymer sorbents where both reactivity and diffusive dynamics can be optimized.
Aminopolymer sorbents are leading candidates for extracting CO2 directly from the atmosphere under ambient conditions. For effective carbon capture, this requires not only that the CO2 actively binds with amine groups of the polymer under low gas concentrations but also that it readily diffuses through the sorbent media to access as many of the amine binding sites as possible. Unfortunately, high reactivity and diffusivity tend to be mutually exclusive properties when it comes to small molecule transport within a polymer, posing a significant materials design challenge. While many reports to date focus on chemical additives or engineering strategies to tackle this trade-off, only a few studies have seriously investigated the underlying chemical and physical properties of the sorbent polymer as a function of its interaction with the relevant sorbate molecules. In this study, we investigate the interplay of polymer-sorbate reactivity and diffusive dynamics of both H2O and CO2 in branched polyethylenimine (PEI) using quasielastic neutron scattering (QENS), infrared spectroscopy, gravimetric uptake, and mechanical dissipation measurements as a function of atmospheric dosing conditions. We uncover an intriguing and previously unreported discrepancy in the diffusive dynamics of PEI dosed with CO2 and H2O vapor at the microscopic and macroscopic length scales. At the macroscopic scale, our mechanical dissipation measurements show that while the exposure to H2O vapor alone always plasticizes the dynamics of PEI, the absorption of CO2, either in the presence of H2O or not, leads to a mechanical stiffening of the PEI. Interestingly, this response differs at the microscopic scale where the diffusive dynamics of the H2O- and/or CO2-dosed samples as quantified by QENS are always enhanced relative to the undosed PEI. This dynamic facilitation is greatest in the presence of H2O vapor alone, consistent with H2O strongly plasticizing the dynamics of PEI. However, the simultaneous exposure to both H2O and CO2 leads to a stiffening of the QENS dynamics at the microscopic scale relative to the hydrated state, signifying local interactions between the CO2 and the polymer. Under these conditions, we also observe a greater amount of CO2 absorbed into the PEI film that is simultaneously exposed to both H2O and CO2 as compared to the film exposed to just CO2, further evidencing a complicated three-way interaction between the H2O, CO2, and PEI. These results are discussed in terms of an absorption process that involves the formation of carbamate ions, the generation of ionic cross-link junctions in the PEI, and changes in the local hydration level of the polymer around the ions. To establish the importance of the carbamate ions in this process, we utilize a methylation reaction to modify the PEI and convert all of the primary and secondary amines into tertiary amines that are incapable of forming carbamates. This considerably diminishes the role of hydrogen bonding in the PEI, enhances the microscopic dynamics of the undosed PEI, and results in diffusive dynamics that do not depend heavily on dosing with H2O and/or CO2. The observations reported here provide insights into the design of next-generation aminopolymer sorbents where both reactivity and diffusive dynamics can be optimized.






