C60-based Multivalent Glycoporphyrins Inhibit SARS-CoV-2 Specific Interaction with the DC-SIGN Transmembrane Receptor
Authors: Jennifer Patino-Alonso, Justo Cabrera-González, Javier Merino, Gema Nieto-Ortiz, Fátima Lasala, Jouma Katati, Carlos H. Bezerra da Cruz, Ajay K. Monnappa, Pablo Mateos-Gil, Ángeles Canales, Iván López-Montero, Beatriz M. Illescas, Rafael Delgado, and Nazario Martín
Journal: Small (2023)
Abstract
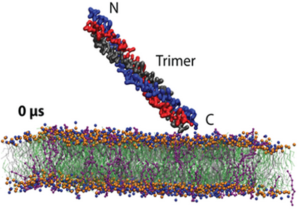
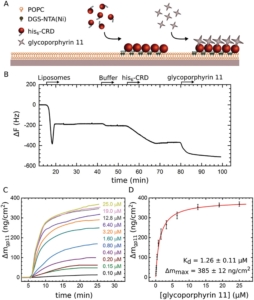
Since WHO has declared the COVID-19 outbreak a global pandemic, nearly seven million deaths have been reported. This efficient spread of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is facilitated by the ability of the spike glycoprotein to bind multiple cell membrane receptors. Although ACE2 is identified as the main receptor for SARS-CoV-2, other receptors could play a role in viral entry. Among others, C-type lectins such as DC-SIGN are identified as efficient trans-receptor for SARS-CoV-2 infection, so the use of glycomimetics to inhibit the infection through the DC-SIGN blockade is an encouraging approach. In this regard, multivalent nanostructures based on glycosylated [60]fullerenes linked to a central porphyrin scaffold have been designed and tested against DC-SIGN-mediated SARS-CoV-2 infection. First results show an outstanding inhibition of the trans-infection up to 90%. In addition, a deeper understanding of nanostructure-receptor binding is achieved through microscopy techniques, high-resolution NMR experiments, Quartz Crystal Microbalance experiments, and molecular dynamic simulations.
You may read the full paper here.
C60-based Multivalent Glycoporphyrins Inhibit SARS-CoV-2 Specific Interaction with the DC-SIGN Transmembrane Receptor