Demonstrating a Quartz Crystal Microbalance with Dissipation (QCMD) to Enhance the Monitoring and Mechanistic Understanding of Iron Carbonate Crystalline Films
Authors: Igor Efimov, Eftychios Hadjittofis, Mustafa M. Alsalem, and Kyra L. Sedransk Campbell
Journal: Langmuir
Abstract:
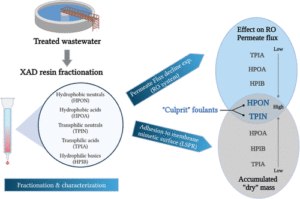
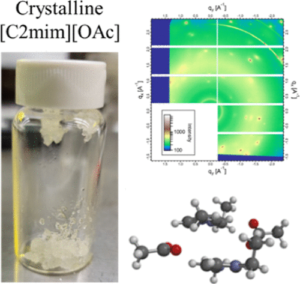
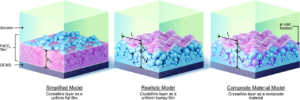 This paper reports the real time monitoring of siderite deposition, on both Au- and Fe-coated surfaces, using the changes in frequency and dissipation of quartz crystal microbalance with dissipation (QCMD). In an iron chloride solution saturated with carbon dioxide, buffered with sodium bicarbonate to pH 6.8, roughly spherical particles of siderite formed within 15 min, which subsequently deposited on the QCMD crystal surface. Imaging of the surface showed a layer formed from particles ca. < 0.5 μm in diameter. Larger particles are clearly deposited on top of the lower layer; these larger particles are >1 μm in diameter. Monitoring of the frequency clearly differentiates the formation of the lower layer from the larger crystals deposited on top at later times. The elastic moduli calculated from QCMD data showed a progressive dissipation increase; the modeling of the solid–liquid interface using a flat approximation resulted in a poor estimation of elastic and storage moduli. Rather, the impedance modeled as a viscoelastic layer in contact with a semi-infinite liquid, where a random bumpy surface with a Gaussian correlator is used, is much more accurate in determining the elastic and storage moduli as losses from the uneven interface are considered. A further step considers that the film is in fact a composite consisting of hard spherical particles of siderite with water in the vacant spaces. This is treated by considering the individual contributions of the phases to the losses measured, thereby further improving the accuracy of the description of the film and the QCMD data. Collectively, this work presents a new framework for the use of QCMD, paired with traditional approaches, to enhance the understanding of crystal deposition and film formation as well as quantify the often evolving mechanical properties.
This paper reports the real time monitoring of siderite deposition, on both Au- and Fe-coated surfaces, using the changes in frequency and dissipation of quartz crystal microbalance with dissipation (QCMD). In an iron chloride solution saturated with carbon dioxide, buffered with sodium bicarbonate to pH 6.8, roughly spherical particles of siderite formed within 15 min, which subsequently deposited on the QCMD crystal surface. Imaging of the surface showed a layer formed from particles ca. < 0.5 μm in diameter. Larger particles are clearly deposited on top of the lower layer; these larger particles are >1 μm in diameter. Monitoring of the frequency clearly differentiates the formation of the lower layer from the larger crystals deposited on top at later times. The elastic moduli calculated from QCMD data showed a progressive dissipation increase; the modeling of the solid–liquid interface using a flat approximation resulted in a poor estimation of elastic and storage moduli. Rather, the impedance modeled as a viscoelastic layer in contact with a semi-infinite liquid, where a random bumpy surface with a Gaussian correlator is used, is much more accurate in determining the elastic and storage moduli as losses from the uneven interface are considered. A further step considers that the film is in fact a composite consisting of hard spherical particles of siderite with water in the vacant spaces. This is treated by considering the individual contributions of the phases to the losses measured, thereby further improving the accuracy of the description of the film and the QCMD data. Collectively, this work presents a new framework for the use of QCMD, paired with traditional approaches, to enhance the understanding of crystal deposition and film formation as well as quantify the often evolving mechanical properties.


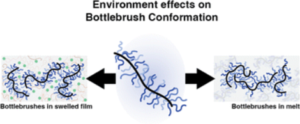 The single-chain physics of bottlebrush polymers plays a key role in their macroscopic properties. Although efforts have been made to understand the behavior of single isolated bottlebrushes, studies on their behavior in crowded, application-relevant environments have been insufficient due to limitations in characterization techniques. Here, we use single-molecule localization microscopy (SMLM) to study the conformations of individual bottlebrush polymers by direct imaging. Our previous work focused on bottlebrushes in a matrix of linear polymers, where our observations suggested that their behavior was largely influenced by an entropic incompatibility between the bottlebrush side chains and the linear matrix. Instead, here we focus on systems where this effect is reduced: in solvent-swollen polymer materials and in systems entirely composed of bottlebrushes. We measure chain conformations and rigidity using persistence length (lp) as side chain molecular weight (Msc) is varied. Compared to a system of linear polymers, we observe greater flexibility of the backbone in both systems. For bottlebrushes in bottlebrush matrices, we additionally observed a scaling relationship between lp and Msc that more closely follows theoretical predictions. For the more flexible chains in both systems, we reach the edge of our resolution limit and cannot visualize the entire contour of every chain. We bypass this limitation by discussing the aspect ratios of the features within the super-resolution images.
The single-chain physics of bottlebrush polymers plays a key role in their macroscopic properties. Although efforts have been made to understand the behavior of single isolated bottlebrushes, studies on their behavior in crowded, application-relevant environments have been insufficient due to limitations in characterization techniques. Here, we use single-molecule localization microscopy (SMLM) to study the conformations of individual bottlebrush polymers by direct imaging. Our previous work focused on bottlebrushes in a matrix of linear polymers, where our observations suggested that their behavior was largely influenced by an entropic incompatibility between the bottlebrush side chains and the linear matrix. Instead, here we focus on systems where this effect is reduced: in solvent-swollen polymer materials and in systems entirely composed of bottlebrushes. We measure chain conformations and rigidity using persistence length (lp) as side chain molecular weight (Msc) is varied. Compared to a system of linear polymers, we observe greater flexibility of the backbone in both systems. For bottlebrushes in bottlebrush matrices, we additionally observed a scaling relationship between lp and Msc that more closely follows theoretical predictions. For the more flexible chains in both systems, we reach the edge of our resolution limit and cannot visualize the entire contour of every chain. We bypass this limitation by discussing the aspect ratios of the features within the super-resolution images.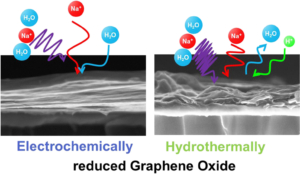 Reduced Graphene Oxide possesses numerous interesting properties, making it one of the most studied materials today. By this way, applications in various fields, including fundamental research, can be found. Nevertheless, the complexity of reduced Graphene Oxide lies in its fabrication process which defines their properties. In this paper, two fabrication methods -electrochemical and hydrothermal reduction of graphene oxide – were compared using physico-chemical and electrogravimetric analysis. Our findings reveal significant morphological differences between the two methods, accompanied by different electrochemical behaviors, when tested in aqueous electrolyte (i.e. 0.5 M Na2SO4). Specifically, electrochemically reduced graphene oxide exclusively involves sodium (whether hydrated or not) in its charge compensation mechanism, whereas hydrothermally reduced graphene oxide also involves proton in sodium sulfate solution.
Reduced Graphene Oxide possesses numerous interesting properties, making it one of the most studied materials today. By this way, applications in various fields, including fundamental research, can be found. Nevertheless, the complexity of reduced Graphene Oxide lies in its fabrication process which defines their properties. In this paper, two fabrication methods -electrochemical and hydrothermal reduction of graphene oxide – were compared using physico-chemical and electrogravimetric analysis. Our findings reveal significant morphological differences between the two methods, accompanied by different electrochemical behaviors, when tested in aqueous electrolyte (i.e. 0.5 M Na2SO4). Specifically, electrochemically reduced graphene oxide exclusively involves sodium (whether hydrated or not) in its charge compensation mechanism, whereas hydrothermally reduced graphene oxide also involves proton in sodium sulfate solution.