Trace Water Effects on Crystalline 1-Ethyl-3-methylimidazolium Acetate
Authors: Ashlee Aiello, John R. Hoffman, Anthony P. Kotula, Lucas Q. Flagg, Ruipeng Li, and Jeremiah W. Woodcock
Journal: J. Phys. Chem. B (2023)
Abstract
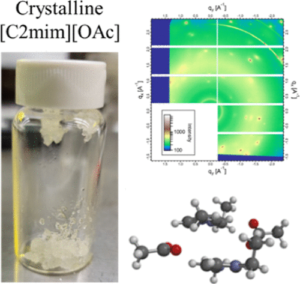
Spontaneous room-temperature crystallization of 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) was observed upon removal of trace water. Sample purity was confirmed using analytical nuclear magnetic resonance spectroscopy to ensure that trace water or other contaminants did not produce this observation. Raman spectroscopy and simultaneous quartz crystal microbalance/infrared spectroscopy measurements were used to study molecular reorganization during crystallization and decrystallization using trace water in the form of atmospheric moisture. These experimental results were supplemented with density functional theory calculations that indicate imidazolium cation ring stacking and side chain clustering with an exclusive arrangement of the acetate anion in the cation ring plane upon water removal. Crystal structure formation was confirmed using two-dimensional wide-angle X-ray scattering. This natural crystallization is attributed to the removal of trace water over extended periods of time and calls attention to the molecular-level role of water in the structure of hygroscopic ionic liquid systems.
You may read the full paper here.
Trace Water Effects on Crystalline 1-Ethyl-3-methylimidazolium Acetate